Mutations of the Tp53 gene occur in a subset of patients with refractory/relapse B-cell lymphoma and confer an exceedingly adverse prognosis. Chimeric antigen receptor-modified (CAR) T-cell immunotherapy represents a novel promising treatment and has achieved impressive anti-tumor responses in patients with refractory or relapsed B cell malignancies. Whether CAR T cell therapy can improve the outcomes of refractory/relapse B-cell lymphoma with Tp53 mutations or high-risk functional classification of Tp53 mutations has not been fully investigated yet.
In phase 2 trial, we evaluated the anti-CAR 19/22 cocktail therapy in patients with relapsed or refractory B-cell lymphoma with Tp53 mutations. The primary endpoint was the percentage of patients with an objective response (complete and partial response). The high-risk Tp53 mutations were defined as missense mutations, 'disruptive' mutations and the 'high-risk group of evolutionary action (EAp53) score. The outcome impact of CAR T cell therapy on high-risk Tp53 mutations were also assessed.
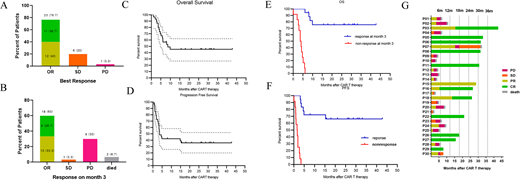
Between September 2016 to January 2020, a total of 30 patients [median age, 44.0 years (range: 28.0 to 61.0)] were enrolled. The primary efficacy analysis showed that 76.7 % (95%CI: 61% to 93%) patients have best objective response(Figure 1A), while 60.0 % (95%CI: 41% to 79%) patients at month three maintained the objective response(Figure 1B). At a median follow-up of 7.95 months (range: 1.13 to 42.76), the 12-months progression-free survival and overall survival rate were 43.33% and 45.33% respectively(Figure 1C-D). The therapy led to similar serious and life-threatening toxic effects with those reported with other CAR T-cell therapies. For all 30 enrolled patients, total 32 Tp53 Mutations were detected in this cohort including 23 missense, 4 splice site mutations, 4 insertions, 2 deletions and 1 nonsense mutation. Two Tp53 mutations were found in 2 patients. 21 patients were assessed with Tp53 FISH and 14 patients (66.66%) had 17p-. There is no difference on ORR,OS and PFS in patients based on three different Tp53-specific scoring systems. The ORR at three months predicts the long-term survival of this group of patients (Figure 1E-F).
CAR19/22 T-cell cocktail therapy can improve the outcome of r/r B-cell lymphoma with high-risk of functional classification of Tp53 mutation. The patients who achieved overall response at month three can greatly benefit from CAR19/22 T-cell cocktail therapy with long-term favorable outcome.
Trial registration: ChiCTR-OPN-16008526.
Key words: B cell lymphoma; CAR T-cell therapy; Tp53 mutation; outcome
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal